Entering the world of Biosimilars

In today's newsletter, we talk about why the Indian pharmaceutical giant Biocon made the headlines a few days ago.
The Story
Three days ago, we noticed a very interesting headline — “Biocon gets US FDA nod to launch insulin glargine, third biologics by Indian firm”
And, truth be told, we couldn’t contain our excitement. Not because we own Biocon shares. We don’t actually. But we’ve been following this story for almost 2 years now.
However, before we get to the headline and tell you why we are so excited — a small detour.
Ever since modern medicine started to emerge post the Industrial Revolution, “simple molecules” have been used to treat most diseases. While these formulations are highly effective against some illnesses, they aren’t particularly effective against more complex diseases like cancer. Our immune system has evolved over millions of years to specifically defend against outside intruders. But cancer isn’t like most diseases. It’s not caused by an invasion of a foreign pathogen. Instead, it’s a byproduct of rogue cells that destroy our bodies from within. To this end, using “simple molecules” to defend against a barrage of mutating versions of our own cells is an exercise in futility. What we probably need is a ‘biologic’ or a complex protein isolated from natural sources that can mimic our immune cells. Maybe that would help us in fighting cancer.
And so, biologics are all the rage these days. But there’s a problem.
Success in this market is deeply intertwined with the research and development process that characterizes the pharmaceutical industry. It might take 5 years for you to develop a new drug and you might still need another 10 years to clinically test the product and get the necessary approvals from the regulatory agencies. This is a capital intensive process and the only way to remunerate the pharma company’s contribution is to protect their investment through patent laws. This way the companies can be incentivised to invest more in research and we can ensure a steady supply of new drugs that could cure the greatest maladies of modern time.
However, once the patent expires, other companies can market their own version of the drug (copycats) if they can figure out how to synthesize it. Consider — Aspirin. It's a simple molecule drug and it’s quite easy to replicate the manufacturing process.
But biologics are harvested from living cells and are often produced using complicated manufacturing processes. Most modern biologics are assembled inside vats — or bioreactors — that house genetically engineered microbes or cell cultures and can often take a whole decade of research to perfect. So replicating the process isn’t exactly a cakewalk.
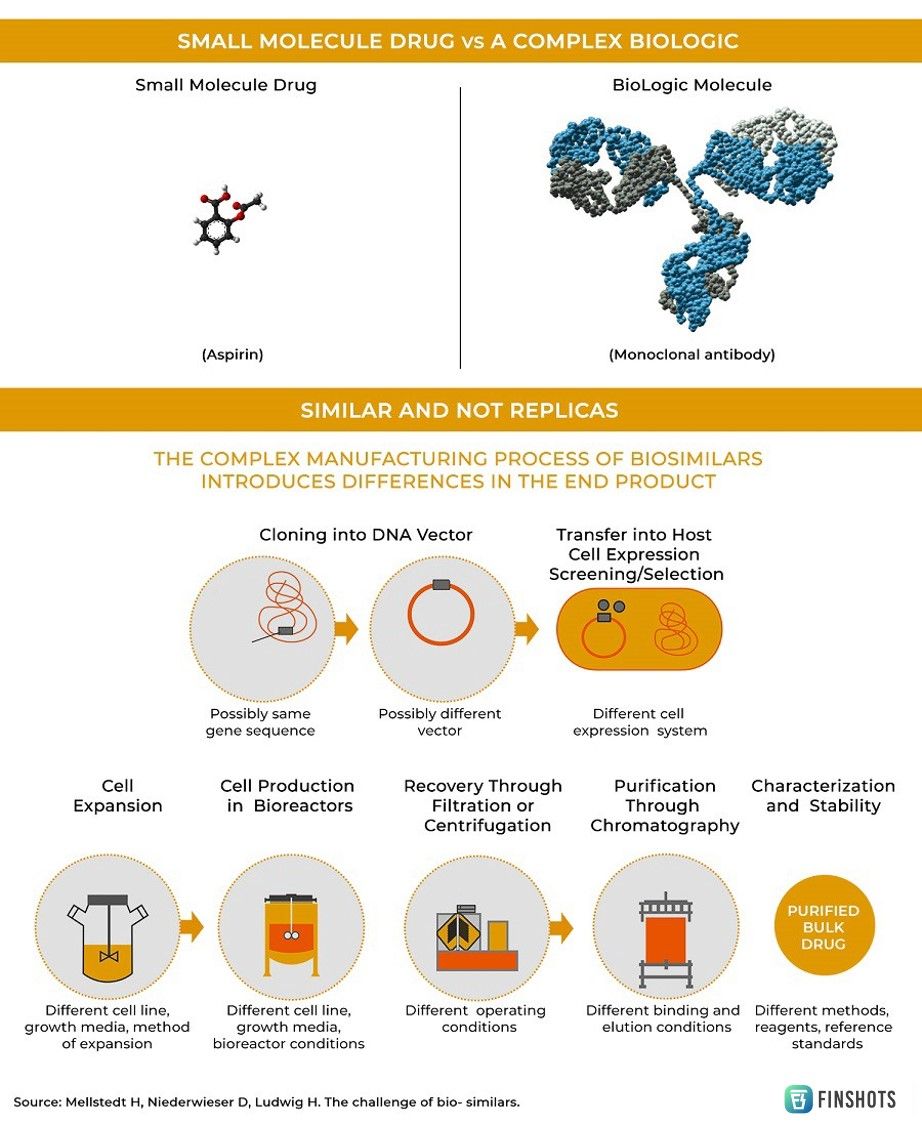
Meaning if you want to market your own version of a “biologic” (once all the patents expire), you need some expertise and India’s Biocon is at the forefront of this revolution. For the past few years, they’ve been building a “biosimilar pipeline” — copycats of famous biologics and they’ve been using it to fight cancer, diabetes, and arthritis.
And since it’s not all that easy for most pharma companies to enter this market, they have a veritable advantage here.
Okay, back to the headline. So it talks about a biosimilar for Insulin Glargine. It’s a form of human insulin. It is used to control blood sugar levels in patients with diabetes and it helps the body by turning all the excess sugar into energy and storing it elsewhere. In fact, two years ago, we talked about Biocon and the Biosimilar revolution. Back then, the company was still trying to get the necessary regulatory approval for this drug. And look where we are now. Amazing!!!
But there’s one other thing. Back then, the drug was already being marketed in other countries. And the news did not garner the same kind of attention. But every time a product is marketed in the US, there’s a special kind of buzz.
Why, you ask?
Well, because the US provides an opportunity like no other. Buying drugs here is expensive and pharmaceutical companies make a killing in the process. While it might not necessarily bode well for consumers, it does provide a lucrative market for potential Indian manufacturers who are looking to sell their products elsewhere.
And this brings us to the headline one last time — the US FDA approval for insulin glargine. Now, despite the untapped potential in the country, the US market is notorious for all kinds of regulatory overhead. The FDA (Food & Drug Administration) takes forever to vet potential drugs and they are very particular about the entire process. So whenever they do approve a drug, it’s cause for celebration.
And that’s why Biocon made the headline 3 days ago.
